Regulatory Affairs is a profession, known as a bridge / a channel of communication, between the Regulatory Agency and the organization seeking approval for new pharmaceutical products.
It plays an important role in Product Life Cycle management stages from “Drug development” to “Commercialization”. For the getting approval of new Products in various countries all over the world, there is an approval procedure; having some set of procedures governed by the respective country regulatory agencies.
New pharmaceutical products registration is required with the complete details of Product and manufacturer in the form of a dossier, The process of registration of Pharmaceutical Drug Substance / Drug Product in US market called as “New Drug Application (NDA)” and for European countries it is called as “Marketing Authorization Application (MAA)”.
 |
| Regulatory Dossier in Pharmaceutical Industry |
❓ What is Regulatory Dossier?
When we find the dossier meaning in pharma, The term “Dossier” is very important in the pharmaceutical industry it is an apex document in the pharma called as Dossier. It is a collection of data and information of pharmaceutical Drug, required to get the registration of drugs / to get marketing approval from various regulatory agencies around the world.
This process is called as “Marketing approval or Registration”, “Marketing Authorization or “Product Licensing”. Dossier has complete history of the Product, subjected for the Marketing Approval and dossier submission is done after the successful completion of the phase III clinical trial.
In simple words “Dossier” may be called as “Registration Dossier”, which contains all technical details regarding the targeted pharmaceutical product, seeking the approval for marketing in the respective country/region.
Technical details expected in the dossier includes administrative information about the manufacturer, Quality details (CMC), non-clinical study details and clinical study details.
Dossier submission done in US market for any new product called as “New Drug Application (NDA)” and for European countries it is called as “Marketing Authorization Application”. For other countries it is called as simply “Registration Dossier;”
“The collection of informative documents / information used as a supporting document for “Regulatory Submission Application”, this grouped / collective documents called as Regulatory Dossier.
❓ What is the purpose of regulatory Dossier Preparation?
Regulatory Dossiers preparation is being performed for the New Drug applications, and it is required to get the Marketing Authorization i.e. License to market the product in particular market.
Simply, regulatory dossier is submitted by the applicant to the regulatory agency in order to obtain approval for marketing the product. These New Drug Applications are termed differently in different regions/countries. Like;
✅ In Japan it is called as JNDA (Japan New Drug Application),
✅ In Canada as NDS (New Drug Submission),
✅ In USA as NDA (New Drug Submission)
✅ In European Countries as MAA i.e. Marketing authorization Application”.
Additionally, it can be prepared for the update on existing submitted dossier, where in update is required or proposed from manufacturer regarding update in any information submitted previously to the respective regulatory agency.
❓ What type of information A Regulatory Dossier has?
Chemistry (CMC), Formulation, Manufacture, Toxicology, Pharmacology, Pharmacokinetics, Clinical studies
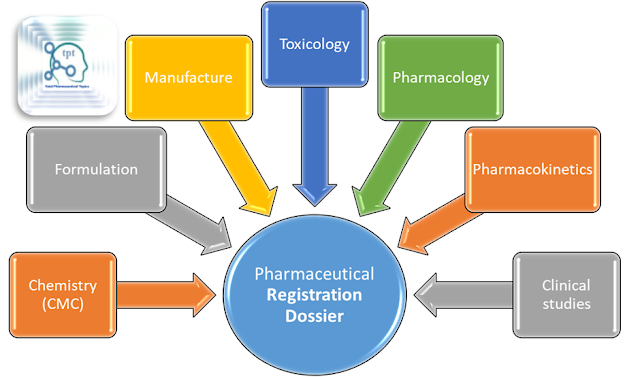 |
| Contents of Regulatory Dossier |
❓ What is format and content for Regulatory Dossier?
The process of dossier submission may vary from one country to another country / region. As the globalization of pharmaceutical industry occurs, it has created the need to harmonization for the development of new pharmaceuticals, as well as the regulatory requirements of various countries.
Thus, a common format of submission has been developed through ICH process, the CTD’s guidance have been developed for Japan, European Union, and United States. Almost Most of the countries have adopted the CTD format.
However, when we deep dive in the subject we found that; basically, there are two types of Regulatory dossier formats are available for the dossier preparation i.e. ICH CTD and ACTD (Asean Common Technical Dossier).
The ICH CTD formats generally used by the ICH countries and developing countries and ACTD format is being used by the group of Asian countries having ten members as Brunei, Cambodia, Indonesia, Lao, Malaysia, Myanmar, Philippines, Singapore, Thailand, Viet Nam.
❓ What is CTD (Common technical Dossier)?
This is a well-organized table of Content for Technical Information on any “Pharmaceutical product” seeking the approval for the marketing in the specific region / market. As we discussed above the organization of ICH CTD is done in 5 modules to gather the information as one.
🔑 Module-1: Regional and Administrative Information
🔑 Module-2: CTD Overview and Summaries
🔑 Module-3: Quality
🔑 Module-4: Non-clinical study details
🔑 Module-5: Clinical study details
There is no difference with respect to the required information on new pharmaceutical drug products, though arrangement of information may be different, like;
🔅 ICH CTD has 5 module within it for the arrangement of information.
🔅 ACTD has 4 Part for the collection of information
For the ICH CTD dossier preparation guidance is published by ICH as ICH-M4, The ICH CTD is organized into five modules. Module 1 is region specific and Modules 2, 3, 4 and 5 are intended to be common for all regions.
In July 2003, the CTD became the mandatory format for new drug applications in the EU and Japan, and the strongly recommended format of choice for NDAs submitted to FDA, United States. This ASEAN Common Technical Dossier (ACTD) is a guideline of the agreed upon common format for the preparation of a well-structured Common Technical Dossier (CTD) application that will be submitted to ASEAN regulatory authorities for the registration of pharmaceuticals and biologics for human use.
Learn More 📗📘📙:
What is Marketing Authorization in Pharmaceutical Industry?
What is the difference between clinical trial & clinical research?
The Evolution of Drug Substance Development
Drug Substance in Pharmaceuticals!! Unveiling Its Vital Role












0 Comments
If you have any doubts, Please contact us