Artificial Intelligence and Automation in Healthcare, these are key step for Revolutionizing Pharma
 |
| Artificial-intelligence-in-pharmaceutical-Industry |
How to define Artificial intelligence and Automation:
Artificial intelligence is a broader concept that covers the simulation of human intelligence by machines, while Automation refers to the use of technology to perform tasks with minimal human intervention.
AI systems are typically more flexible and capable of learning and adapting, while automation systems are often simpler and more static. To understand the difference between these two terms refer below tabulated details;
|
Aspect |
Artificial
Intelligence (AI) |
Automation |
|
Definition |
Simulation
of human intelligence processes by machines |
Use
of technology to perform tasks with minimal human intervention |
|
Capabilities |
Mimics
human cognitive functions like learning and decision-making |
Performs
tasks based on pre-defined rules |
|
Flexibility |
More
flexible and adaptable, capable of learning and improving over time |
Relatively
static, performs specific tasks without much variation |
|
Complexity |
Often
more complex, requiring advanced algorithms and processing capabilities |
Can
be simpler, with tasks like mixing, granulation, and packaging |
|
Applications
in pharmaceuticals |
Drug
discovery, clinical trial optimization, personalized medicine |
Manufacturing
processes, quality control, supply chain management |
|
Examples |
AI
algorithms analyzing data to identify drug candidates, optimizing
clinical trials |
Robotic
systems for manufacturing, packaging, and logistics |
How to utilized Artificial intelligence and Automation in Pharmaceutical Industry?
Artificial intelligence can be utilized in various ways to enhance compliance with regulatory requirements:
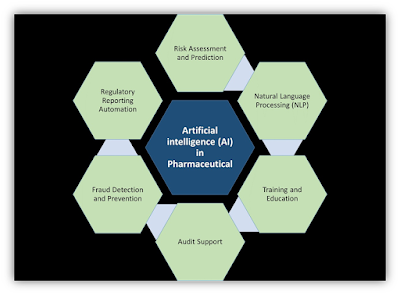 |
| Artificial-intelligence-in-pharmaceutical-Industry-2 |
1. Automated Monitoring and Reporting: AI-powered systems can continuously monitor vast amounts of data and flag any inconsistencies or deviations from regulatory standards. This helps organizations stay compliant in real-time and enables them to take prompt corrective actions.
2. Risk Assessment and Prediction: AI algorithms can analyze historical data to identify patterns and predict potential compliance risks. By anticipating issues before they arise, organizations can proactively address them, reducing the likelihood of regulatory violations.
3. Natural Language Processing (NLP): NLP algorithms can parse through regulatory documents, contracts, and other legal texts to extract relevant information. This helps ensure that organizations understand and adhere to the intricate details of regulatory requirements.
4. Data Privacy Compliance: AI tools can assist in ensuring compliance with data privacy regulations by automatically anonymizing sensitive information, implementing access controls, and monitoring data usage to prevent unauthorized disclosures.
5. Regulatory Reporting Automation: AI systems can streamline the process of generating regulatory reports by extracting and consolidating data from various sources, reducing the time and resources required for compliance reporting.
6. Training and Education: AI-powered e-learning platforms can provide personalized training programs tailored to specific regulatory requirements, ensuring that employees are well-informed and equipped to adhere to compliance standards.
7. Fraud Detection and Prevention: AI algorithms can analyze transactional data to detect anomalies indicative of fraudulent activities, helping organizations comply with regulations related to financial transparency and anti-money laundering.
8. Audit Support: AI can facilitate audits by organizing and presenting relevant data in a structured format, making it easier for auditors to assess compliance and identify areas for improvement.
Overall, AI offers significant potential to enhance regulatory compliance by
automating routine tasks, improving risk management, and ensuring timely and
accurate adherence to regulatory standards.
----------------------------------------------------------------------------------------------------------------------
Automation in pharmaceutical is being utilized in different ways to improve efficiency, accuracy, and safety in various processes. Some common types of automation are;
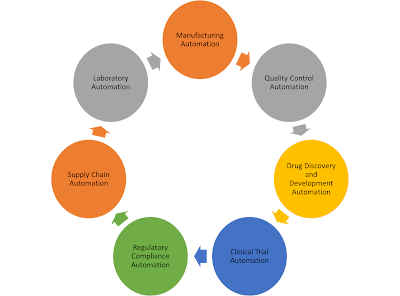 |
| Automation in Pharmaceutical industry |
1. Manufacturing Automation: This includes automated processes for drug formulation, blending, granulation, tablet pressing, encapsulation, packaging, and labelling.
2. Laboratory Automation: Laboratory automation involves automated systems for sample preparation, analysis, and testing, such as high-throughput screening, liquid handling, chromatography, and spectrophotometry.
3. Quality Control Automation: Automated systems are used for quality control testing, including microbiological testing, stability testing, and analytical testing to ensure product quality and compliance with regulatory requirements.
4. Supply Chain Automation: This includes automated systems for inventory management, warehousing, and distribution to optimize supply chain processes and ensure timely delivery of pharmaceutical products.
5. Regulatory Compliance Automation: Automation is used to streamline regulatory compliance processes, including electronic document management, data integrity verification, and audit trail generation to meet regulatory requirements.
6. Clinical Trial Automation: Automation is used to streamline various aspects of clinical trials, including patient recruitment, data collection, monitoring, and reporting to improve efficiency and accuracy in clinical research.
7. Drug Discovery and Development Automation: Automation is used in various stages of drug discovery and development, including virtual screening, molecular modeling, high-throughput screening, and combinatorial chemistry to accelerate the drug discovery process.
Overall, automation plays a crucial role in improving productivity, efficiency, and quality in the pharmaceutical industry while ensuring compliance with regulatory requirements and maintaining patient safety.
Learn More.......
Pharmaceutical Drug substance (API) and Drug Product: Definition
Drug Substance and Drug Product Manufacturing Flow: Discovery to Delivery
Drug Substance in Pharmaceuticals!! Unveiling Its Vital Role












0 Comments
If you have any doubts, Please contact us