Introduction to Clinical Trial?
When we talk about the pharmaceutical development, Clinical trial is a significant term. What is clinical trial? This term can be defined as; the clinical study of health and illness in people.
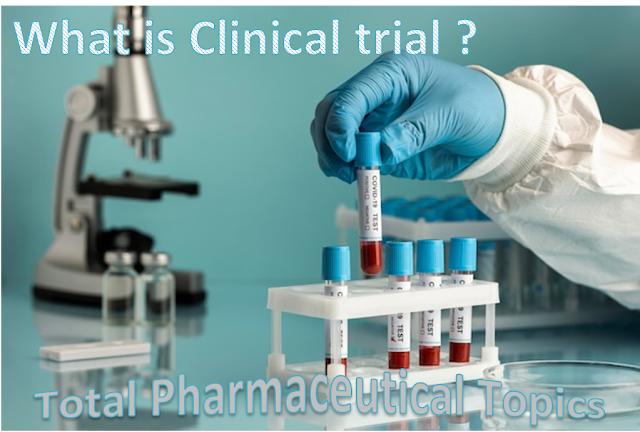 |
| Fig. 1 : What is Clinical Trial? |
In previous article pharmaceutical development, we understand the Path to Pharmaceutical Development via “Drug Substance and Drug Product Manufacturing Flow: Discovery to Delivery. In continuation we will have an idea about Clinical Trial. There are two core words in clinical research: i.e. Observational Studies and Clinical Trials.
Observation studies involves the monitoring activity of people in normal conditions. Scientists those are involve in this observation study; gathers informations on people and compare changes over time. Observational studies do not test a medical intervention, such as a drug or device, but may help identify new treatments or prevention strategies to test in clinical trials.
Clinical trials are the unsung heroes of modern medicine, quietly shaping the landscape of healthcare. These trials are the pivotal stages that new drugs & treatments must pass through before they reach your medicine cabinet.
In this blog article, we'll embark on a journey through the world of clinical trials, shedding light on some questions i.e. what they are? how they work? & why they are crucial to our well-being?. By the end of this article, we will have a better understanding of the fascinating & intricate world of clinical trials.
What is Clinical Trial?
Fundamentally, the clinical trial is a precisely planned investigation; that seeks to assess the efficacy &safety of a novel medication, medical technology/therapy. The results of these trials are crucial in establishing the safety & efficacy of novel interventions for use in humans. It offers the empirical data that healthcare practitioners and regulators need to make well-informed decisions regarding patient care.
 |
| Fig. 2 : Clinical Trial: Precisely Planned Investigation |
These studies follow specific protocols, recruit participants according to eligibility criteria, and collect empirical data to determine the intervention's impact on health and illness in people. Clinical trials are essential in advancing medical science, providing evidence-based information for healthcare decision-making, and ensuring that new treatments are safe and effective before they are widely adopted for patient care.
04 Phases of Clinical Trials :
Clinical trials are typically divided into four phases, each serving a specific purpose:
Phase
1: Safety First
Phase 1 of
Clinical trials are the initial testing ground for a new intervention. These
trials involve a small group of healthy volunteers to evaluate the safety,
dosage and potential side effects. They help researchers, to determine how the
human body processes the new treatment.
Phase
2: Efficacy and Side Effects
Phase 2 of
Clinical trials expand the test group to include individuals with the condition
or disease the treatment is intended for. These trials aim to assess the
intervention's effectiveness and monitor any side effects.
Phase
3: Large-Scale Testing
Phase 3
of Clinical trials involve a larger and more diverse group of patients.
Researchers compare the new treatment to existing treatments or placebos to
determine its safety and effectiveness on a broader scale.
Phase
4: Post-Market Surveillance
After a
treatment is approved and available to the public, Phase 4 trials continue to
monitor its long-term effects, safety, and efficacy in real-world conditions.
Why Clinical Trials Matter?
Clinical trials have a profound impact on healthcare and society as a whole. They're the engines behind the advancement of medical science, providing healthcare providers with crucial, evidence-based data to make informed decisions for patient care.
Clinical trials rigorously assess the safety of new treatments, ensuring that patients are not exposed to unnecessary risks.
These trials drive medical innovation, leading to new treatments, therapies, and drugs that improve patient outcomes. Clinical trial results are the foundation of evidence-based medicine, helping healthcare providers make informed decisions about patient care.
Regulatory bodies, like the FDA in the United States, rely on clinical trial data to determine if a treatment should be approved for public use. Participation in clinical trials gives patients access to cutting-edge treatments and contributes to the advancement of medical science.
Participation in a Clinical Trial
Choosing
to take part in a clinical studys can be a very fulfilling and significant
choice. By participating in a clinical trial, you not only increase the
possibility that your health may improve but also advance medical science. It's
an opportunity to receive state-of-the-art care that may not yet be accessible
to the general public, which can be very helpful if you have a difficult
medical conditions. Clinical trials offer a closely monitored, encouraging
atmosphere that guarantees your safety at various stage. In addition to being a
personal adventure, your involvement benefits society at large by assisting
researchers in their search for more effective therapies and solutions.
General
steps for Participation in a clinical trial
1. Find a Trial: Use online resources or
consult your healthcare provider to find a clinical trial relevant to your
condition.
2. Screening: Once you identify a
trial, you'll undergo a screening process to determine if you meet the
eligibility criteria.
3. Informed Consent: If you qualify,
you'll receive detailed information about the trial and must provide informed
consent to participate.
4. Trial Participation: If you decide
to participate, you'll follow the trial protocol, which may include taking the
investigational treatment and attending regular check-ups.
5. Regular Monitoring: Throughout the
trial, you'll be closely monitored to track the treatment's effects and any
potential side effects.
6. Contribution to Science: Your
participation contributes valuable data to the research, helping researchers and
society as a whole.
Ethical Considerations: Clinical trials must adhere to strict ethical guidelines, to ensure the safety & well-being of participants. These guidelines; include informed consent, patient privacy & an independent review board to oversee the trial's conduct.
In Brief
Clinical trials are the backbone of pharmaceutical development procedure; They bridge the gap between scientific discovery & effective patient care, offering hope to those with health conditions and driving innovation in medicine.
With the understanding the various phases of
Clinical Trials, importance, and ethical considerations of clinical trials, it
is appreciable, the crucial role it plays in advancing healthcare.
So, the next time you hear about a clinical trial, you'll know that it's a example of hope for patients and a symbol of progress in the world of pharmaceuticals.
Learn More:
The Evolution of Drug Substance Development
Pharmaceutical Quality Management System: Excellence in Drug Manufacturing
Drug Substance in Pharmaceuticals!! Unveiling Its Vital Role
Drug Substance and Drug Product Manufacturing Flow: Discovery to Delivery












0 Comments
If you have any doubts, Please contact us