In the ever-evolving landscape of the pharmaceutical industry, ensuring the quality and reliability of processes is paramount. One crucial aspect that stands at the forefront is the process validation—a meticulous journey that guarantees the efficiency and safety of pharmaceutical manufacturing.
 |
| 10 Key Steps for Successful Process Validation in Pharma |
In this article, we will unravel the intricacies of process validation in pharma, exploring the 10 key steps that pave the way for success. From understanding critical process parameters to navigating FDA regulations, let's embark on this journey together.
Step 1: Setting the Foundation –
Step 2: Navigating the Regulatory Landscape –
Step 3: Identifying Crucial Factors -
Step 4: Crafting the Blueprint –
Step 5: Unveiling the Lifecycle –
Step 6: Documenting the Journey –
Step 7: Ensuring Compliance –
Step 8: Mastering Precision –
Step 9: Integrating Statistical Wisdom –
Step 10: Achieving Continuous Improvement –
---------------------------------------------------------------------------------------------------------------------------------------
Step 1: Setting the Foundation –
Understanding Pharmaceutical Process Validation Before diving into the specifics, let's establish a solid foundation by comprehending the essence of pharmaceutical process validation. This step involves recognizing the significance of validation in maintaining product quality and patient safety.
 |
| Fig. 01: Setting
the Foundation Successful Process Validation in Pharma |
At its core, process validation is the systematic assessment and documentation of a manufacturing process to ensure that it consistently produces a product of predetermined quality. It's not merely about meeting regulatory standards but also about optimizing processes for efficiency, reducing risks, and ensuring the reliability of every pharmaceutical product that reaches the market.
For Understanding Basics on Process validation Learn More.......;
@ What is Validation? Basic overview
@What is Process Validation? What are the Types of validation?
---------------------------------------------------------------------------------------------------------------------------------------
Step 2: Navigating the Regulatory Landscape –
FDA Regulations for Process Validation; The pharmaceutical industry operates within a stringent regulatory framework & adherence to FDA regulations is non-negotiable.
 |
| Fig. 02: Navigating the Regulatory Landscape for Successful Process Validation in Pharma |
The FDA, as the regulatory authority overseeing pharmaceuticals in the United States, plays a pivotal role in shaping the Pharmaceutical process validation Guideline. Adhering to these regulations is non-negotiable for any pharmaceutical manufacturer. The FDA regulations for process validation provide a framework that emphasizes the importance of a well-defined and controlled manufacturing process. Key elements include the validation master plan, a comprehensive document that outlines the overall strategy for the validation process.
Have an overview on Basics on regulatory aspects Learn More.....
@ Pharmaceutical regulatory authorities All overthe world
---------------------------------------------------------------------------------------------------------------------------------------
Step 3: Identifying Crucial Factors –
Critical Process Parameters At the heart of successful process validation lies an in-depth understanding of critical process parameters (CPP). Uncover the factors that can significantly impact the quality, safety, and efficacy of pharmaceutical products, and learn how to manage and control them effectively.
 |
| Fig. 03: Identifying Crucial Factors for Successful Process Validation in Pharma |
Critical process parameters (CPPs) are the variables that have a direct impact on the quality of the pharmaceutical product. Identifying and monitoring these parameters are central to successful process validation. From temperature and pressure to time and mixing speed, each parameter must be precisely controlled to ensure the desired product quality. Understanding the critical process parameters sets the stage for effective validation, enabling manufacturers to establish and maintain control over the entire production process.
---------------------------------------------------------------------------------------------------------------------------------------
Step 4: Crafting the Blueprint –
A validation master plan is like the blueprint for ensuring everything in a process works smoothly. It covers what needs validating, how to do it, and ensures all steps meet regulatory standards, ensuring products are consistently top-notch.
 |
| Fig. 04: Crafting the Blueprint for Successful Process Validation in Pharma |
Validation Master Plan (VMP), A standard Validation Master Plan Template is required to serves as the roadmap for validation journey. Explore the essential elements of a VMP, detailing how it provides a comprehensive overview of the entire validation process, ensuring a systematic and organized approach.
---------------------------------------------------------------------------------------------------------------------------------------
Step 5: Unveiling the Lifecycle –
Process Validation Lifecycle Process validation is not a one-time event; it's a continuous lifecycle. Walk through each phase of the process validation lifecycle, from design and qualification to continued monitoring and improvement.
Understand how this cyclical approach ensures ongoing compliance and product quality. The process validation lifecycle is a continuous journey that begins with the design phase and extends through the entire life of a product.
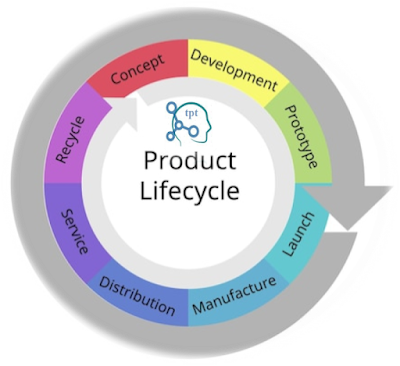 |
| Fig. 05: Unveiling the Lifecycle for Successful Process Validation in Pharma |
It comprises three stages: Stage 1 - Process Design, Stage 2 - Process Qualification, and Stage 3 - Continued Process Verification. Each stage plays a crucial role in ensuring the ongoing success of process validation. This section will provide a detailed exploration of each stage, highlighting the key activities and considerations at every step.
Have an overview on Basics on Lifecycle approach, Learn More.....
@ Whatis Product Lifecycle Management..? An Overview
@ Drug Substance and Drug Product Manufacturing Flow: Discovery to Delivery
---------------------------------------------------------------------------------------------------------------------------------------
Step 6: Documenting the Journey –
Validation Protocols and Reports Effective documentation is the backbone of successful process validation. Learn the art of creating validation protocols and reports that not only satisfy regulatory requirements but also serve as valuable tools for process improvement.
 |
| Fig. 06: Documenting the Journey for Successful Process Validation in Pharma |
Validation protocols are detailed plans outlining how a process will be tested to ensure it meets quality standards. Reports document the results of these tests, confirming that the process consistently produces the desired outcomes. Together, they provide a transparent record of the validation process, demonstrating compliance with regulatory requirements and ensuring the reliability of product quality.
Learn More.....
Validationstudy: Why Three batch for validation study?
---------------------------------------------------------------------------------------------------------------------------------------
Step 7: Ensuring Compliance –
Validation, Deviations and CAPA Even with meticulous planning, deviations can occur. Explore the process of managing validation deviations and implementing Corrective and Preventive Actions (CAPA) to maintain compliance and enhance overall process reliability.
 |
| Fig. 07: Documenting the Journey for Successful Process Validation in Pharma |
Continuous process verification involves ongoing monitoring and control to ensure that the process remains in a state of control throughout commercial production. By adhering to these validation principles, organizations can enhance product quality, reduce the risk of defects, and ultimately achieve and maintain compliance with regulatory requirements, fostering confidence in the reliability of their processes and products.
---------------------------------------------------------------------------------------------------------------------------------------
Step 8: Mastering Precision –
Mastering precision in the context of process validation is a critical aspect that underscores the meticulous control and optimization of manufacturing processes to ensure consistent and accurate outcomes, which involves detailed mapping of process parameters and their acceptable ranges.
Validation Equipment Qualification The equipment used in pharmaceutical manufacturing plays a pivotal role in process success. Dive into the details of equipment qualification, understanding how precise calibration and validation ensure the accuracy and reliability of your manufacturing processes.
 |
| Fig. 08: Mastering Precision for Successful Process Validation in Pharma |
By mastering precision in process validation, industries can enhance product quality, reduce the likelihood of defects, and adhere to regulatory requirements. This commitment to precision not only ensures the reliability of manufacturing processes but also fosters confidence in the reproducibility of high-quality products.
---------------------------------------------------------------------------------------------------------------------------------------
Step 9: Integrating Statistical Wisdom –
Statistical Process Control (SPC); SPC is a powerful tool for maintaining consistent quality. Discover how statistical methods and data analysis contribute to the ongoing monitoring and control of critical process parameters. Statistical process control Software plays an important role in the execution of the SPC like minitab, IQMS manufacturing ERP, WinSPC etc.
 |
| Fig. 09: Integrating Statistical Wisdom for Successful Process Validation in Pharma |
Integrating statistical wisdom in process validation involves using statistical methods to gather insights, analyze data, and enhance decision-making. It's about harnessing the power of statistics to ensure precision, reliability, and compliance in manufacturing processes. By applying statistical tools, we gain a deeper understanding of variability, optimize parameters, and maintain consistent product quality. This approach fosters a data-driven culture that not only meets regulatory standards but also enhances overall efficiency and confidence in the validation process.
---------------------------------------------------------------------------------------------------------------------------------------
Step 10: Achieving Continuous Improvement –
Continuous Process Verification The journey doesn't end with initial validation. Embrace the concept of continuous process verification, a proactive approach that fosters ongoing improvement and ensures the long-term success of pharmaceutical processes.
 |
| Fig. 10: Achieving Continuous Improvement for Successful Process Validation in Pharma |
Achieving continuous improvement in process validation means consistently refining and enhancing manufacturing processes. It involves a proactive approach to identifying and implementing better methods, minimizing errors, and optimizing efficiency. This ongoing commitment ensures that products meet high standards, comply with regulations, and reflects a dedication to excellence in quality and reliability
---------------------------------------------------------------------------------------------------------------------------------------
In Brief........
By incorporating these 10 key steps into the pharmaceutical process validation journey, it will not only meet regulatory requirements but also elevate the overall quality and reliability of manufacturing processes. Here's to achieving excellence in every step of the way!
We Team TPT Hope this comprehensive article certainly helped to enrich the knowledge bucket of your mind..!! Keep reading………. J












0 Comments
If you have any doubts, Please contact us